- Joined
- Apr 7, 2016
- Messages
- 678
- Reaction score
- 2,269
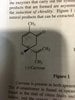
Which of the following statements are valid for the isomers of carvone and limonene?
I. Both limonene and carvone have four possible stereoiosmers each.
But the answer explains that both carvone and limonene have one chiral center, so they will each have two possible stereoiosmers (enantiomers). Making statement I valid.
Am I missing something here?
I. Both limonene and carvone have four possible stereoiosmers each.
But the answer explains that both carvone and limonene have one chiral center, so they will each have two possible stereoiosmers (enantiomers). Making statement I valid.
Am I missing something here?