- Joined
- Mar 24, 2007
- Messages
- 1,724
- Reaction score
- 9
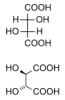
I have two lingering confusions about stereoisomers. The best compound to help explain the first confusion is tartaric acid, so I've included a link that shows its both the d,l and meso stereoisomers.
http://en.wikipedia.org/wiki/Tartaric_acid
1. the 2 carbons (bound to the OHs) are connected by a sigma bond and we all know rotation around sigma bonds happens (which can cause conformational isomerism etc). If you have 180* rotation about that C-C bond for either the d or l stereoisomer won't you get the corresponding meso-compound if not why? and if you do get the mesocompound then are the different stereoisomers of tartaric acid also conformers?
2. For (cis/trans) geometric isomerism in cyclic compounds.
CIS = similar substituents on same side of the ring
TRANS = similar substituents on opposite sides of the ring.
My confusion here is what does same side of the ring mean.
For dimethylcyclohexane is the cis isomer when the CH3 groups are on C1 & C2 respectively and trans would have them on C1 and C4? Or does cis mean that the CH3 are both above or below the plane of the ring (irrespective of which C that are bound to) and trans means that one CH3 is above the plane of the ring and the other below the plane. In other words for cyclic compounds, do you only need 2D to determine cis/trans or do you need 3D?
http://en.wikipedia.org/wiki/Tartaric_acid
1. the 2 carbons (bound to the OHs) are connected by a sigma bond and we all know rotation around sigma bonds happens (which can cause conformational isomerism etc). If you have 180* rotation about that C-C bond for either the d or l stereoisomer won't you get the corresponding meso-compound if not why? and if you do get the mesocompound then are the different stereoisomers of tartaric acid also conformers?
2. For (cis/trans) geometric isomerism in cyclic compounds.
CIS = similar substituents on same side of the ring
TRANS = similar substituents on opposite sides of the ring.
My confusion here is what does same side of the ring mean.
For dimethylcyclohexane is the cis isomer when the CH3 groups are on C1 & C2 respectively and trans would have them on C1 and C4? Or does cis mean that the CH3 are both above or below the plane of the ring (irrespective of which C that are bound to) and trans means that one CH3 is above the plane of the ring and the other below the plane. In other words for cyclic compounds, do you only need 2D to determine cis/trans or do you need 3D?