- Joined
- Jul 30, 2016
- Messages
- 177
- Reaction score
- 39
I figured it would be better to have all the Section Bank Qs easy to find since I have seen the same Q's come up multiple times and this will make it easier to find them all.
I am having trouble figuring out the reasoning behind this Q, #46 in the AAMC C/P Section Bank
Q 46
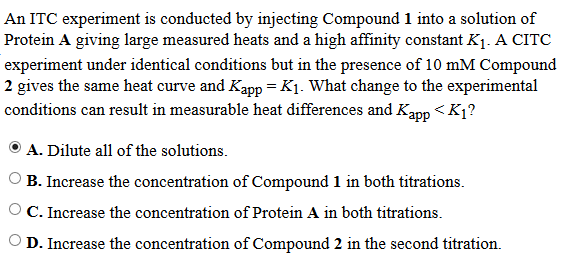
They say the answer is D, and I went with B. I am trying to figure out the proper reasoning, since the AAMC explanations suck so much as to be useless.
The only information given in the passage is this equation which relates the Kapp of a substrate for an enzyme to its K value via comparison with the K value of another substrate for that same enzyme.
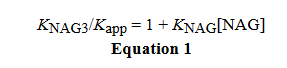
In this Equation Kapp refers to the apparent (from the experiment) association constant of NAG for the enzyme, KNAG3 represents the affinity between the same enzyme and NAG3, and KNAG represents the actual (if i got that right) affinity of NAG for the enzyme.
Rearranging the equation I can get Kapp = (KNAG3) / (1 + KNAG[NAG])
The Q wants us to determine what would lower Kapp. Looking at equation, 1 I can lower the Kapp by several ways:
Lower the affinity of the enzyme for the substrate, NAG3 (i.e. KNAG3)
Raise the affinity of the enzyme for substrate NAG (i.e. KNAG)
Raise the concentration of [NAG]
Thus, by adding more of the 2nd substrate, NAG, I can lower Kapp. But I am nto sure if this is the reasoning I am supposed to use. The explanation does not mention the equation at all!

So, I am looking for alternative reasons to explain why D is correct given the question information.
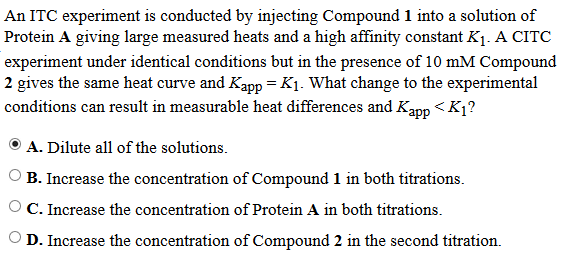
The way I read this is:
Experiment 1 = Substrate 1 (aka compound 1) + Enzyme (Protein A)
K1 is resulting affinity value
Experiment 2 = Substrate 2 (aka compound 2) + Enzyme (protein A)
Kapp is the resulting affinity value, and Kapp = K1
We need a way to lower Kapp, so aside from the equation, which says if I add more of the 2nd substrate (NAG in the original equation) I will lower the Kapp value for the substrate, how else does adding more of Substrate 2 to the 2nd titration lower its Kapp?
Am I wrong, or did the AAMC just chose to ignore the equation in its explanation for some reason? Does Kapp in the Q stem NOT refer to the apparent association constant between compound 2 and the protein? Does the 2nd titration include protein A, compound 2 AND compound 1? The text says "under identical conditions but in the...." so maybe they mean protein A and compound 1 again with compound 2 added?
That I could also see why adding more of compound 2 could interfere with the Kapp for compound 1, but I thought Kapp refers to the apparent affinity of compound 2 for the protein.
This Q has me baffled if the equation is NOT the way to the right answer. Seems the AAMC would try to at least explain the correct answer, lord knows they cannot be bothered to describe the wrong answers.
I thank the MCAT gods for non-AAMC materials.
Rant over, thank you all!
I am having trouble figuring out the reasoning behind this Q, #46 in the AAMC C/P Section Bank
Q 46
They say the answer is D, and I went with B. I am trying to figure out the proper reasoning, since the AAMC explanations suck so much as to be useless.
The only information given in the passage is this equation which relates the Kapp of a substrate for an enzyme to its K value via comparison with the K value of another substrate for that same enzyme.
In this Equation Kapp refers to the apparent (from the experiment) association constant of NAG for the enzyme, KNAG3 represents the affinity between the same enzyme and NAG3, and KNAG represents the actual (if i got that right) affinity of NAG for the enzyme.
Rearranging the equation I can get Kapp = (KNAG3) / (1 + KNAG[NAG])
The Q wants us to determine what would lower Kapp. Looking at equation, 1 I can lower the Kapp by several ways:
Lower the affinity of the enzyme for the substrate, NAG3 (i.e. KNAG3)
Raise the affinity of the enzyme for substrate NAG (i.e. KNAG)
Raise the concentration of [NAG]
Thus, by adding more of the 2nd substrate, NAG, I can lower Kapp. But I am nto sure if this is the reasoning I am supposed to use. The explanation does not mention the equation at all!
So, I am looking for alternative reasons to explain why D is correct given the question information.
The way I read this is:
Experiment 1 = Substrate 1 (aka compound 1) + Enzyme (Protein A)
K1 is resulting affinity value
Experiment 2 = Substrate 2 (aka compound 2) + Enzyme (protein A)
Kapp is the resulting affinity value, and Kapp = K1
We need a way to lower Kapp, so aside from the equation, which says if I add more of the 2nd substrate (NAG in the original equation) I will lower the Kapp value for the substrate, how else does adding more of Substrate 2 to the 2nd titration lower its Kapp?
Am I wrong, or did the AAMC just chose to ignore the equation in its explanation for some reason? Does Kapp in the Q stem NOT refer to the apparent association constant between compound 2 and the protein? Does the 2nd titration include protein A, compound 2 AND compound 1? The text says "under identical conditions but in the...." so maybe they mean protein A and compound 1 again with compound 2 added?
That I could also see why adding more of compound 2 could interfere with the Kapp for compound 1, but I thought Kapp refers to the apparent affinity of compound 2 for the protein.
This Q has me baffled if the equation is NOT the way to the right answer. Seems the AAMC would try to at least explain the correct answer, lord knows they cannot be bothered to describe the wrong answers.
I thank the MCAT gods for non-AAMC materials.
Rant over, thank you all!
