7
752779
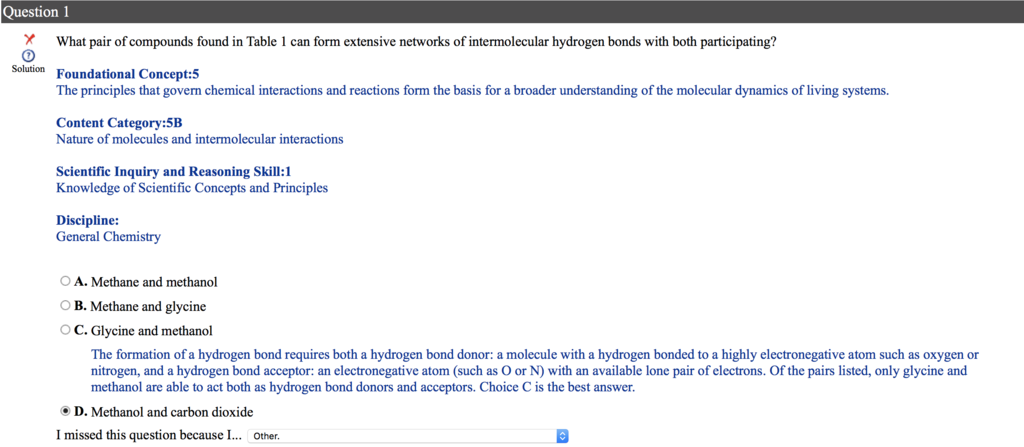
Hello, Can you some please help me. I'm a little confused. I took general chemistry 10 years ago. I understand why I got the question wrong. However I don't understand why isn't Florine is listed as the most electronegative atom in the periodic table, even though the choices given don't have any Florine atom.
Much thanks.
Much thanks.
