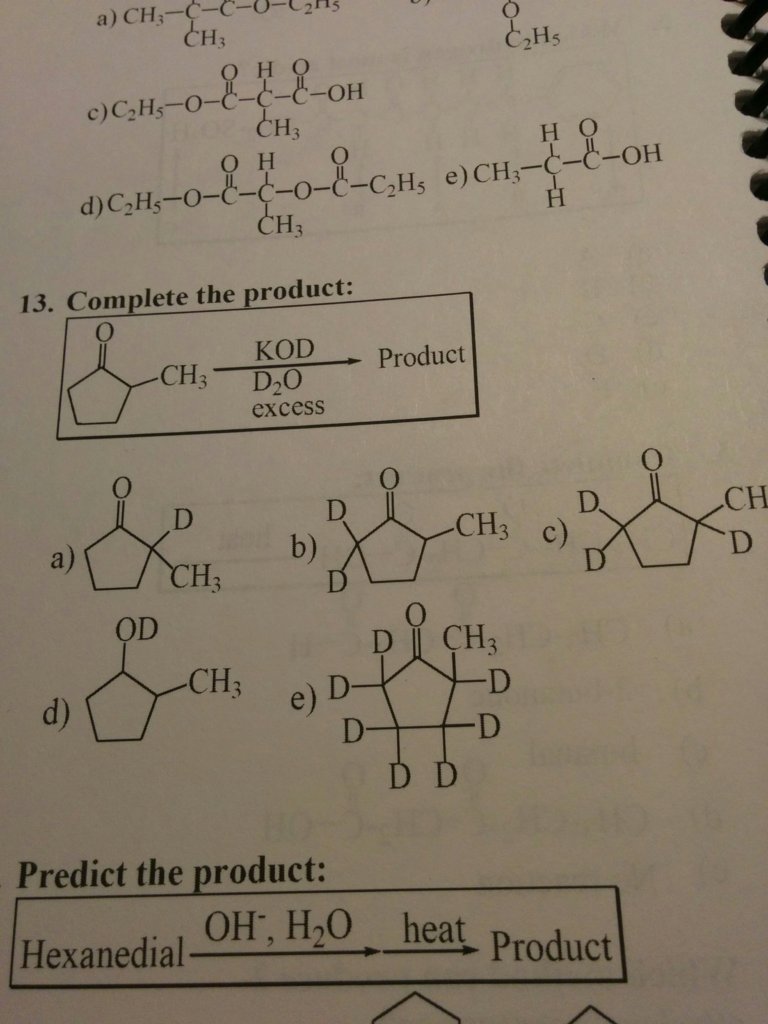
Do we always treat the D2O as a D-D or do we ever treat it like a D-OD (alcohol/water)? In the reaction pictured below, we treat it as a D2 and add to every alpha hydrogen. However, if we treated it as a D-OD like ch3oh wouldn't it only add to the most acidic hydrogen? Sorry I always get confused on these deuterion problems
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
D2O as D2 or D-OD?
- Thread starter 510586
- Start date
- Joined
- Mar 12, 2005
- Messages
- 4,799
- Reaction score
- 3,490
Do we always treat the D2O as a D-D or do we ever treat it like a D-OD (alcohol/water)? In the reaction pictured below, we treat it as a D2 and add to every alpha hydrogen. However, if we treated it as a D-OD like ch3oh wouldn't it only add to the most acidic hydrogen? Sorry I always get confused on these deuterion problems View attachment 193597
You have missed a very important concept. The DO- is a base and will remove all the possible acidic protons and replace with D's . Usually it is not so easy to stop at only one. For problems like this also assume excess. In real life this is the way many organic chemistry compounds are deuterated.
Hope this helps
Dr. Romano
So the koh isn't why it is basic?You have missed a very important concept. The DO- is a base and will remove all the possible acidic protons and replace with D's . Usually it is not so easy to stop at only one. For problems like this also assume excess. In real life this is the way many organic chemistry compounds are deuterated.
Hope this helps
Dr. Romano
- Joined
- Mar 12, 2005
- Messages
- 4,799
- Reaction score
- 3,490
So the koh isn't why it is basic?
You would not use KOH when you want to put on a D, this might prevent all the Hydrogens from being replaced with D's.
Sorry I meant the KOD not KOH making it basic. Would it ever be D2O with D3O+?You would not use KOH when you want to put on a D, this might prevent all the Hydrogens from being replaced with D's.
- Joined
- May 10, 2015
- Messages
- 708
- Reaction score
- 614
Do we always treat the D2O as a D-D or do we ever treat it like a D-OD (alcohol/water)? In the reaction pictured below, we treat it as a D2 and add to every alpha hydrogen. However, if we treated it as a D-OD like ch3oh wouldn't it only add to the most acidic hydrogen? Sorry I always get confused on these deuterion problems View attachment 193597
This is very similar to the halogenation reactions at the alpha carbons. This is simply alpha duteration.
Note that the reagent in KOD. This is the same as KOH but you are using an isotope of hydrogen called duterium. They will react in the same way. KOD will turn the carbonyl into an enolate. D2O (which is the same as h2o) will then add duterium ions to the alpha carbon just like when you generate an enolate via a base like LDA and then add X2. Just like alpha-halogenation, alpha-duteration will "over duterate". So every alpha hydrogen will turn into a duterium ion
Oh ok i keep getting D2O confused with the alcohols that add to the alpha position. Like with LDA or KOH but adding ch3oh instead of a halogen.This is very similar to the halogenation reactions at the alpha carbons. This is simply alpha duteration.
Note that the reagent in KOD. This is the same as KOH but you are using an isotope of hydrogen called duterium. They will react in the same way. KOD will turn the carbonyl into an enolate. D2O (which is the same as h2o) will then add duterium ions to the alpha carbon just like when you generate an enolate via a base like LDA and then add X2. Just like alpha-halogenation, alpha-duteration will "over duterate". So every alpha hydrogen will turn into a duterium ion
- Joined
- Mar 12, 2005
- Messages
- 4,799
- Reaction score
- 3,490
Sorry I meant the KOD not KOH making it basic. Would it ever be D2O with D3O+?
Yes 510586......D3O+ would also do the job. However, when done in base it goes much much better.
An excellent review of this is presented in the Carey text book.
Hope this helps..
Dr. Romano
- Joined
- May 10, 2015
- Messages
- 708
- Reaction score
- 614
Oh ok i keep getting D2O confused with the alcohols that add to the alpha position. Like with LDA or KOH but adding ch3oh instead of a halogen.
Yes but as you probably know, if you first generate an enolate via base and then add an alcohol, you will get a hemiacetal/acetal.
I think the only reason you were confused was because the question used duterium
Oh OK so it would never add an od or something because that isn't acetal. Thanks!Yes but as you probably know, if you first generate an enolate via base and then add an alcohol, you will get a hemiacetal/acetal.
I think the only reason you were confused was because the question used duterium
Similar threads
- Replies
- 5
- Views
- 2K
- Replies
- 3
- Views
- 856
- Replies
- 6
- Views
- 2K

