- Joined
- Feb 29, 2016
- Messages
- 157
- Reaction score
- 50
Bio
If I get a question that a woman carries a sex-linked gene do i automatically assume it's recessive unless she expresses the phenotype then homozygous?
G chem
Is this a type of ? that i'll get on the DAT? if yes then how do i solve it?
What volume of HCl was added if 20 ml of 1 M NaOH is titrated with 1 M HCl to produce a pH=2?
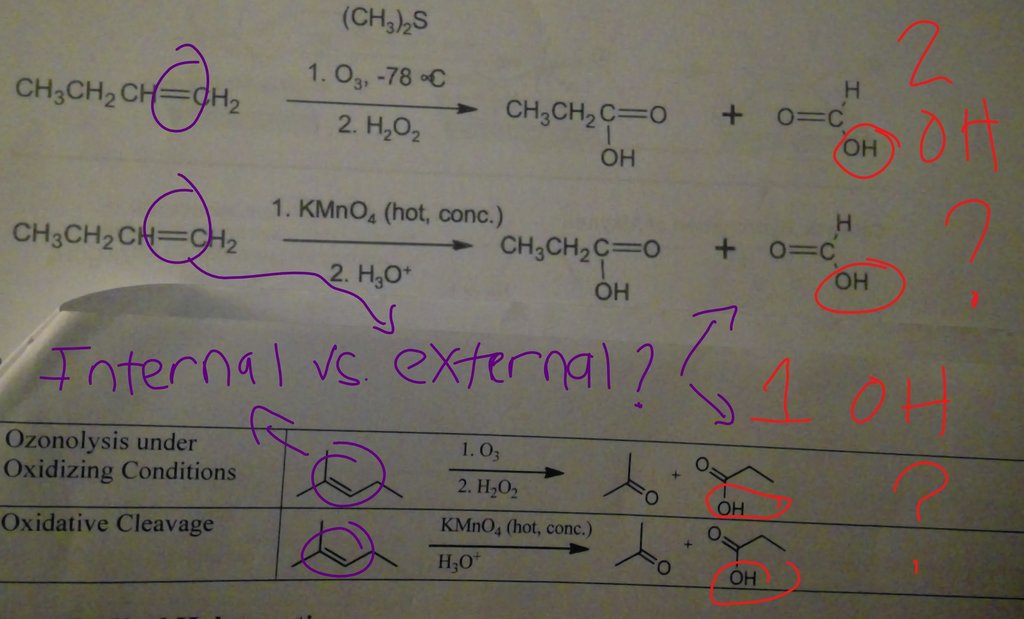
Orgo please check attchment
If I get a question that a woman carries a sex-linked gene do i automatically assume it's recessive unless she expresses the phenotype then homozygous?
G chem
Is this a type of ? that i'll get on the DAT? if yes then how do i solve it?
What volume of HCl was added if 20 ml of 1 M NaOH is titrated with 1 M HCl to produce a pH=2?
Orgo please check attchment
